How Accurate Is Radiocarbon Dating?
Radiocarbon ages less than 3,500 years old are probably accurate. However, before accepting any radiocarbon date, one should know how the technique works, its limitations, and its assumptions. One limitation is that the radiocarbon technique dates only material that was once part of an animal or plant, such as bones, flesh, or wood. It cannot date rocks directly. To understand the other capabilities and limitations of radiocarbon dating, we must understand how it works and consider the flood.
Most carbon atoms weigh 12 atomic mass units. However, roughly one in a trillion carbon atoms weighs 14 atomic units. This carbon is called carbon-14. It is also called radio carbon because it is radio active (but not dangerous). Half of it will decay in about 5,730 years to form nitrogen. Half of the remainder will decay in another 5,730 years, and so on.
Cosmic radiation striking the upper atmosphere converts about 21 pounds of nitrogen each year into radiocarbon (carbon-14). Most carbon-14 quickly combines with oxygen to form radioactive carbon dioxide, which then spreads throughout the atmosphere. Plants take in carbon dioxide, incorporating in their tissues both carbon-14 (unstable) and normal carbon-12 (stable) in the same proportion as they occur in the atmosphere . Carbon-14 then moves up the various food chains to enter animal tissue—again, in about the same ratio carbon-14 has with carbon-12 in the atmosphere.
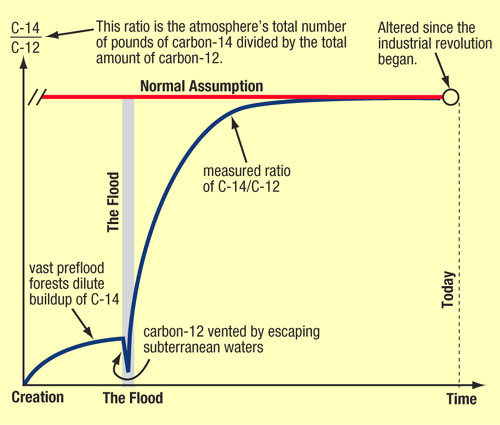
When a living thing dies, its radiocarbon loss (decay) is no longer balanced by intake, so its radiocarbon steadily decreases with a half-life of 5,730 years. If we knew the amount of carbon-14 in an organism when it died, we could attempt to date the time of death. The key questions then are: “Has the atmospheric ratio of carbon-14 to carbon-12 changed in the past, and if so, why and how much?” The assumption usually made, but rarely acknowledged, is that the ratio of carbon-14 to carbon-12 in the atmosphere before the industrial revolution has always been the same—about one in a trillion. Actually, that ratio may have been quite different.
For example, a worldwide flood would uproot and bury preflood forests. Afterward, less carbon would be available to enter the atmosphere from decaying vegetation. With less carbon-12 to dilute the carbon-14 continually forming from nitrogen in the upper atmosphere, the ratio of carbon-14 to carbon-12 in the atmosphere would increase. If the atmosphere's ratio of carbon-14 to carbon-12 has doubled since the flood and we did not know it, radiocarbon ages of things living soon after the flood would appear to be one half-life (or 5,730 years) older than their true ages. If that ratio quadrupled, organic remains would appear 11,460 (2 x 5,730) years older, etc. Therefore, a “radiocarbon year” would not correspond to an actual year.
As explained in recent measurements show that the ratio of carbon-14 to carbon-12 has been building up in the atmosphere. However, for the last 3,500 years, the increase in the ratio has been extremely slight.
Radiocarbon dating of vertical sequences of organic-rich layers at 714 locations worldwide has consistently shown a surprising result. Radiocarbon ages do not increase steadily with depth, as one might expect. Instead, they increase at an accelerating rate. In other words, the concentration of carbon-14 is unexpectedly low in the lower organic layers. As one moves to higher and higher layers, this concentration increases, but at a decreasing rate.
Tree-ring dating allows us to infer how the atmospheric concentration of carbon-14 changed in the past. Some types of trees growing at high elevations with a steady supply of moisture will reliably add only one ring each year. In other environments, multiple rings can be added in a year. A tree ring's thickness depends on the tree's growing conditions, which vary from year to year. Some rings may show frost or fire damage. By comparing sequences of ring thicknesses in two different trees, a correspondence can sometimes be shown. Trees of the same species that simultaneously grew within a few hundred miles of each other may have similar patterns. Trees of different species or trees growing in different environments have less similar patterns.
Claims are frequently made that wood growing today can be matched up with some scattered pieces of dead wood so that tree-ring counts can be extended back more than 8,600 years. This may not be correct. These claimed “long chronologies” begin with either living trees or dead wood that can be accurately dated by historical methods. This carries the chronology back perhaps 3,500 years. Then the more questionable links are established based on the judgment of a tree-ring specialist. Sometimes “missing” rings are added. Each tree ring's width varies greatly around the tree's circumference. Also, parts of a ring may be dead wood. Standard statistical techniques could establish how well the dozen supposedly overlapping tree-ring sequences fit. However, tree-ring specialists have refused to subject their judgments to these statistical tests and would not release their data, so others can do these statistical tests. Even less reliable techniques claim to be able to calibrate carbon-14 dating back 26,000 years or more.
Several laboratories in the world are now equipped to perform a much improved radiocarbon dating procedure. Using atomic accelerators, a specimen's carbon-14 atoms can now be actually counted, giving a more precise radiocarbon date with even smaller samples. The standard, but less accurate, radiocarbon dating technique only counts the rare disintegrations of carbon-14 atoms, which are sometimes confused with other types of disintegrations.
This new atomic accelerator technique has consistently detected at least small amounts of carbon-14 in every organic specimen—even materials that evolutionists claim are millions of years old, such as coal. This small, consistent amount is found so often among various specimens that contamination can probably be ruled out. Ancient human skeletons, when dated by this new “accelerator mass spectrometer” technique, give surprisingly recent dates. In one study of eleven sets of ancient human bones, all were dated at about 5,000 radiocarbon years or less!
Radiocarbon dating of supposedly very ancient bones should provide valuable information. Why is such testing rare? Researchers naturally do not waste money on a technique that destroys their specimen and provides no specific age. Therefore, most researchers do not radiocarbon date any organic specimen they think is older than 100,000 years, even if it still contains carbon. All carbon-14 that was once in anything older than 100,000 radiocarbon years would have decayed; its age could not be determined. However, if a bone an evolutionist thinks is a million years old contains any detectable carbon-14, the bone is probably less than 100,000 radiocarbon years.
Bones or other organic remains that contain enough carbon and are believed by evolutionists to be older than 100,000 years will be shown to be relatively young in blind radiocarbon tests. This prediction, first published in the 6th Edition (1995), p. 157, has now been confirmed.
Very precise measurements now show that most fossils—regardless of presumed “geologic age”—have roughly the same ratio of carbon-14 to carbon-12. (This includes fossil fuels: coal, oil, and methane.) Therefore, this former life must have been living at about the same time—less than 100,000 years ago. Because almost all fossils are preserved in water deposited sediments, all this former life was probably buried in a fairly recent, gigantic flood.
Radiocarbon dating is becoming increasingly important in interpreting the past. However, one must understand how it works and especially how a flood affected radiocarbon dating. Radiocarbon ages less than 3,500 years are probably accurate. Ages around 40,000 radiocarbon years, which are typical of coal, have much younger true dates—near the time of the flood, roughly 5,000 years ago.
Chapter 4: What about carbon dating? 
- What about carbon dating? How does the carbon ‘clock’ work? Is it reliable?
- What does carbon dating really show? What about other radiometric dating methods?
- Is there evidence that the earth is young? See Study Guide, Lesson 4
Creation Answers Book Creation.com
Return to top of page
Genesis 1:1 (In the beginning God created the heaven and the earth.)
Back to Anycalculator Creation Moments 